Dispensing is one of the most critical activities which is performed in pharmaceutical industries.
The importance of dispensing can be understood by the fact that dispensing is the first process that is carried out in pharmaceutical industries.
What is Dispensing in Pharma
The dispensing process is also known as weighing and as the name indicates dispensing or weighing is the activity or process where weighing of all the ingredients of a formulation is performed.
Explanation
Dispensing is the process in which we weigh all the excipients and active pharmaceutical ingredients according to the manufacturing order or dispensing order. The dispensing order is also known as the process order.
Pre Requisite For Dispensing
To start a dispensing process following are the prerequisites,
- Process Order
- Released Material
- Dispensing Area
- Line Clearance
- Standard Procedure
- Trained Staff
Process Order
A manufacturing order or process Order is issued by the planning department for a specific product.
it includes the list of all the excipients and APIs that are going to be added in a batch formulation.
For the dispensing process, an approved process order must be available to the raw material store.
Material Release Status
After receiving the process order the status of all the materials is checked. All the materials mentioned in the process order must be released from the quality control department & available in stock.
Nowadays this activity is done by various ERP systems & a specific shop order is released after the above-mentioned checks.
Dispensing Area
For dispensing a batch dispensing booth must be available in pharma & it must be separated from other manufacturing areas to prevent chances of cross–
contamination.
The dispensing booth must have separate personal & material entry.
Classification Of Dispensing Area
The cleanroom classification of the dispensing area must be the same as where the batch will be manufactured or processed after dispensing.Example
If we are going to dispense the batches of tablet or capsule dosage forms that are manufactured in ISO class 8 then the dispensing area class will also be ISO class 8.
If we are going to dispense batches for sterile areas which require ISO class 5 then the class of dispensing both will also be ISO class 5.
Line clearance
After receiving the process order & ensuring the release status & availability of all the materials the next step is the line clearance.
During line clearance, the dispensing area is properly checked for cleanliness & free from previous labels or products.
The calibration status of weight balance, temperature & humidity along with differential pressure is also checked.
If a pre-dispensing area is available then all the materials in the pre-dispensing area are verified according to process order.
Read More about line clearance.
Standard Dispensing Procedure
An SOP for the dispensing process must be present clearly mentioning how the process of dispensing will be performed.
Trained Staff
To carry out the dispensing process trained staff including operator & qualified personnel must be available.
Pre-Dispensing
Pre-dispensing is an activity where we place all the materials outside the dispensing booth in a designated area before starting the process of dispensing.
Many pharmaceutical industries do not follow the process of pre-dispensing, meaning they do not place all the materials in full form required for a batch in pre pre-dispensing area.
They take every material one by one from the racks, dispense it, and then place it back to its original position.
Explanation
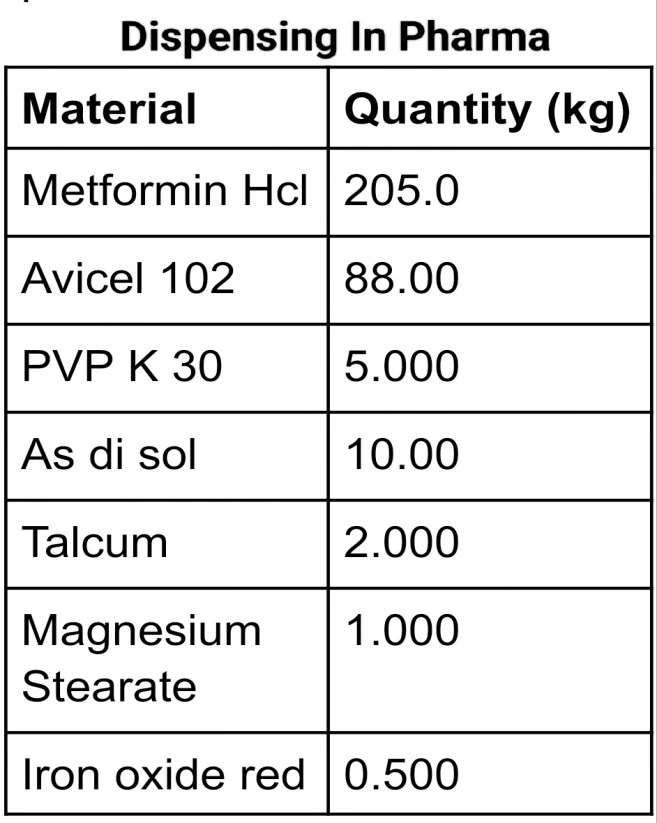
Suppose we want to dispense a batch where we have a total of 7 Excipients having the following quantities.
| Material | Quantity (kg) |
| Metformin Hcl | 205.0 |
| Avicel PH 102 | 88.00 |
| PVP K 30 | 5.000 |
| As di sol | 10.00 |
| Talcum | 2.000 |
| Magnesium Stearate | 1.000 |
| Iron oxide red | 0.500 |
Metformin comes in packaging of 50 kg, Avicel & all remaining comes in packaging of 20 kg.
So for pre-dispensing, we will place the following numbers of containers of the above materials in the pre-dispensing area.
Metformin HCl 5 containers( 4 for full supply and 1 for loose material dispensing),
Avicel 102, 5 containers (4 for full & one for loose), and one container for each of the remaining materials.
During line clearance, the quality assurance pharmacist & dispensing pharmacist will check the identification name,lots of material, expiry & retest dates of all above materials according to the process order.
When the dispensing process starts,the operator will supply materials one by one to the dispensing booth for weighing from the pre-staging area.
Dispensing Procedure
- First of all take the approved process order & check the availability and release status of all the materials to be dispensed.
- Place all the materials in the pre-dispensing area and take line clearance from the quality assurance officer.
- Trained staff is deployed for the dispensing process after proper gowning.
- The material is weighed in a clean polythene bag & for this first of all check the tare weight of the polythene bag, mention it on the label then write net weight according to process order and mention the gross weight on the label & fix it on the polythene bag.
- The label also contains the material name, batch number, lot number of material & signing options.
- The material container to be dispensed is shifted from the pre-dispensing area to the dispensing area through the material entry airlock system.
- The quality assurance officer & dispensing pharmacist verify the material name, lot number, expiry & retest date.
- The container is opened in a dispensing booth under LAF & material is weighed on a calibrated weighing balance with the help of SS scoops.
- The weight is verified by the qualified staff & polythene is sealed with a tie clip or rubber band.
- The dispensed material is placed in a container labeled with dispensed material & the container from which the material is dispensed is sent back to its original rack place.
- In the same manner all the materials are dispensed according to the process order.
Storage Of Dispensed Materials
- The dispensed batch is shifted to the storage area of the raw material store after completion of the dispensing process.
- The storage area is a lock & key area with controlled temperature & humidity.
- All the materials of the dispensed batch are placed on the same pellet (off the floor & away from the wall) with a proper identification label & its log is filled.
- This batch is then handed over to the production department on demand after proper verification & documentation.
Dispensing Sequence
The good sequence for dispensing materials in the dispensing area is as follows,
- First of all, dispense excipients.
- After excipients dispense active pharmaceutical ingredients.
- After active pharmaceutical ingredients dispense flavoring agents.
- After flavors dispense colorants.
Logical Reasoning
What should be the order of dispensing?
According to my observation many pharmaceutical industries first dispense active pharmaceutical ingredients then dispense excipients and some pharmaceutical industries dispense excipients first followed by APIs.
Which dispensing order is good and which is not? Now we will discuss it according to logical reasoning.
Good Dispensing Sequence
First of all, we should dispense the excipients and then we should dispense the active pharmaceutical ingredients.
Reasons
Whenever we expose or open any container in the dispensing booth & perform weighing activity, its particles may remain suspended in the area and when we dispense active pharmaceutical ingredients then these particles may settle down in API containers.
The majority of the excipients used in various batches are the same so when these excipients will mix with APIs containers then there will be no risk as excipients are inert.
If we dispense API first and then dispense the excipients, API-suspended particles will contaminate the excipient.
As we know many excipients are used in different products and when we use contaminated excipients in other products it may result in serious harm, especially in the case of potent drugs.